ATOMS #1: LIGHT AND MATTER
- WACADEMY
- Dec 4, 2023
- 4 min read
Updated: Dec 17, 2023
ATOMS #1: LIGHT AND MATTER
Chemical bond formation is driven by interactions between electrons and nuclei.
In order for us to understand electrons, we need to comprehend quantum mechanics.
ELECTROMAGNETIC RADIATION

^whenever we discuss electrons, we must discuss electromagnetic radiation (click below for its definition). I personally understand EM radiation as energy produced by moving charged particle such as electrons. They inherently carry energy as they travel.

Definitions of electromagnetic radiation from various sources
For the purpose of the chemistry exam, it is important to know and be able to utilize the formula shown below inside the blue box.
1st IMPORTANT FORMULA YOU NEED TO KNOW for this section:

c = speed of light ~ 3.0 x 10^8 m/s (in vacuum)
λ = wavelength (lambda); measured in meters (m)
v = frequency (nu); measured in Hertz (Hz) or 1/seconds (NOTE you can also use the symbol "f" as well)

Notice that magnetic field (B) and electric field (E) are perpendicular to each other, but they are in phase. "In phase" means they go "up" together and "down" together.

^In a graph like this, you can determine its wavelength in several ways as shown above: they should all give you the same value.
Distance from crest to crest (crest = highest point on graph) - top double-sided arrow
Distance from node to node - middle double-sided arrow
Distance from trough to trough (trough = lowest point on graph) - bottom double-sided arrow
ELECTROMAGNETIC SPECTRUM

^EM spectrum gives you the range of all types of EM radiation.
First off, the different colours represent varying Energy, Frequency, and Wavelengths.
"Although all electromagnetic waves travel at the speed of light in a vacuum, they do so at a wide range of frequencies, wavelengths, and photon energies."
"A steady current produces a steady magnetic field, while a changing current induces a changing magnetic field, which in turn creates a changing electric field, resulting in electromagnetic waves. The electric and magnetic fields of an electromagnetic wave are"

^ROY G BIV is the acronym you want to memorize as it tells you the order from lowest energy to highest energy.
ROY G BIV = Red, Orange, Yellow, Green, Blue, Indigo, Violet.
Red has the lowest energy and violet has the highest energy.
Now let's see how these different energy values relate to frequencies and wavelengths:

2nd IMPORTANT FORMULA YOU MUST KNOW for the purpose of chem exam:
E = Energy
h = Planck's constant (a universal constant) = 6.62607015×10−34 joule-hertz−1
f = frequency (recall we used "v" previously, but f and v can be used interchangeably)
Based on this equation, we know that Energy is DIRECTLY RELATED TO Frequency. Directly related means that if E increase, f also increases and vice versa. Since the violet colour has the highest energy, it also has the highest frequency based on the formula.
Now, bringing the 1st formula back, if we rearrange the formula "c=λ f", we know that frequency and wavelength are INVERSELY RELATED, meaning if one increases, the other decreases.
This means the violet colour, which has highest Energy and highest Frequency, has the smallest or shortest wavelength.
ENERGY IS QUANTIZED

^this is an interesting slide that shows that Energy is quantized, meaning it increases or decreases in DISCRETE (or specific) amounts. This means that Energy, just like matter, is DISCONTINUOUS.
PHOTOELECTRIC EFFECT

^I know the slide looks confusing with lots of words, but let me break it down for you:
first off, Photoelectric effect is the phenomenon where when light strikes the metal surface with enough energy, electron is ejected out of the metal.
A given photon of energy has a certain amount of energy according to E = hf (or hv)
Since this photon of energy is striking the electron (or photoelectron), it must have HIGHER energy than the MINIMUM energy required to release an electron (Φ, known as work function) --> in other words, work function is the energy an electron possesses represented by Φ = hf (or hv)
Any leftover energy is conserved as KINETIC ENERGY
Taking point #3 a bit further, for electrons to be ejected, the photon must have greater frequency (v) than that of the electron (v0)
Stopping voltage (Vs) is defined as the voltage or negative potential required to stop the moving electrons
3rd IMPORTANT FORMULA YOU NEED TO KNOW:

Ephoton = energy of photon
Φ = work function (=minimum energy required to eject an electron off metal surface)
K.E. electron = kinetic energy of a given electron
hv = Planck's constant x frequency (This is from E = hv)
hv0 = Planck's constant x frequency of electron
me = mass of electron
u = speed of electron
The formula allows you to calculate the Kinetic Energy and Speed of the electron that has been emitted from the metal plate by a photon of light or energy.
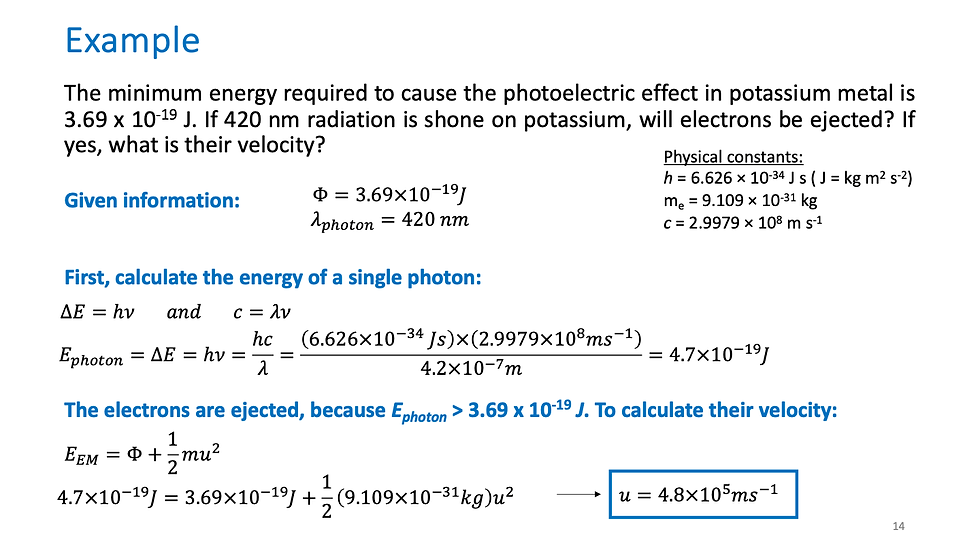
^Now that we have the basics down, let's put these into practice. Try on your own, then compare answers given in the slide.
ATOMIC EMISSION SPECTRA


^Now, scientists discovered that when we heat up atoms, they emit light at discrete (or specific yet distinct) frequencies, thus the term "atomic emission SPECTRA".
4th and 5th IMPORTANT FORMULAS to memorize for this section:
(note that these formulas are specifically for electrons within Hydrogen atoms):

E = Energy (produced by the transition of electron from one quantum state to another in a Hydrogen atom)
RH = Rydberg constant = 2.179 x 10^-18 J nf = final principal shell of e- (aka final quantum state)
ni = initial principal shell of e- (aka initial quantum state)
n = principal shell of e- (quantum state)

^similar idea, but this formula allows you to find the Energy of electron within a Hydrogen atom based on the value of n.

^Now that you have basic concepts down, try this example for practice.




Comments